The mass number A of an atom is the total number of its nuclear constituents, or nucleons, as the protons and neutrons are collectively called. The mass number is placed before and above the elemental symbol, thus 238 U. The mass number gives a useful rough figure for the atomic mass; for example, 1 H = 1.00814 atomic mass units (amu), 238 U = 238.124 amu, and so on.
Mass Numbers - How to find Mass Numbers
The mass number is established by rounding the atomic weight to the nearest whole number. The Periodic Table with Atomic Masswill give you the atomic weight, or atomic mass, of the elements. The chemical properties of an element are determined by its Atomic Number not its Mass Number which is why atomic numbers are shown on the Periodic table whilst Mass Numbers are not. Mass numbers equal the total number of heavy, or massive, particles in the nucleus. Subtracting the Atomic number from the Mass Number equals the number of neutrons in the nucleus.
- The mass number is the sum of the number of protons and neutrons in an atom. It is a whole number. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. It is a decimal number.
- Massachusetts State Lottery Site.
Mass Numbers = Atomic Weight of Element, rounded to nearest whole number
Number of Neutrons = Mass Number - Atomic Number
Mass Numbers
Mass Numbers - The Mass Numbers of all of the elements
So, if we know the number of protons and neutrons in an atom we can determine the mass number. The unique chart below has been created by www.elementalmatter.info and details all of the elements in the Periodic table, the numbers of protons, the numbers of neutrons and the mass numbers of atoms which relate to the elements.
Mass numbers - Examples of Mass Numbers
The following examples provide details of how to calculate the mass number.
- Example 1 - mass number of Gold: The element Gold (Symbol Au) has the Atomic Number of 79. The number of protons in atom of gold is therefore 79. Gold has the Atomic Mass weight of 196.97. Round to the nearest whole number. The mass number of gold is therefore 197.
- Example 2 - mass number of Silver: The element Silver (Symbol Ag) has the Atomic Number of 47. The number of protons in atom of silver is therefore 47. Silver has the Atomic Mass weight of 107.87. Round to the nearest whole number. The mass number of silver is therefore 108.
- Example 3 - mass number of Neon: The element Neon (Symbol Ne) has the Atomic Number of 10. The number of protons in atom of neon is therefore 10. Neon has the Atomic Mass weight of 20.18. Round to the nearest whole number. The mass number of neon is therefore 20.
Mass Numbers = Atomic Weight of Element, rounded to nearest whole number
Mass numbers - Chart of Mass Numbers
The details all of the elements in the Periodic table, the numbers of protons, the numbers of neutrons and the mass numbers of atoms which relate to the elements in the Periodic Table.
Chart of Mass Numbers

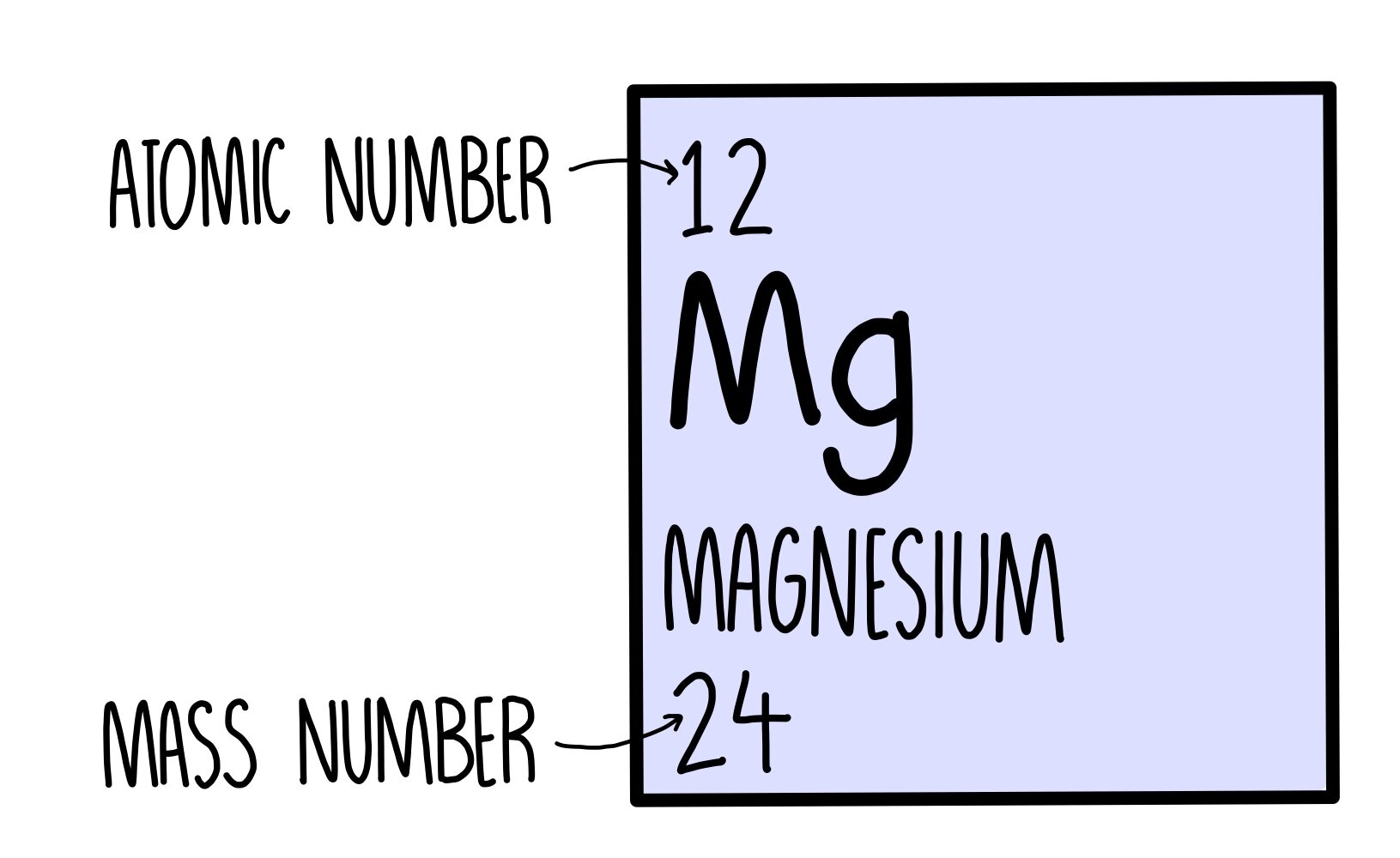
| Atomic Number | Name of Element | Symbol of Element | Mass Numbers | Name of Element |
| 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 | Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon Cesium Barium Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon Francium Radium Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Ununbium Ununtrium Ununquadium Ununpentium Ununhexium Ununseptium Ununoctium | H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo | 1 4 7 9 11 12 14 16 19 20 23 24 27 28 31 32 35 40 40 40 45 48 51 52 55 56 58 58 64 65 70 73 75 79 80 84 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 133 137 139 140 141 144 145 150 152 157 159 163 165 167 169 173 175 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 223 226 227 232 231 238 237 244 243 247 247 251 252 257 258 259 262 261 268 263 264 269 268 272 273 277 286 289 288 292 292 293 | Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon Cesium Barium Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon Francium Radium Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Ununbium Ununtrium Ununquadium Ununpentium Ununhexium Ununseptium Ununoctium |
| Atomic Number | Name of Element | Symbol of Element | Mass Numbers | Name of Element |
Mass Numbers Game
Chart of Mass Numbers
Mass Number Of Sodium
Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus.
The mass number allows enable us to tell the difference between elements with a different number of neutrons, called isotopes.
The mass number is a count and is unitless.

Formula to calculate mass number.
Mass Number Of Carbon
Example 1:
Mass Numbers Game Winning Number
The hydrogen atom doesn’t have a neutron but it has 1 proton, so the mass number of hydrogen is 1.
Example 2:
Oxygen atom has 8 protons and 8 neutrons. Determine the mass number.
Thus, oxygen atom mass number is 16.
